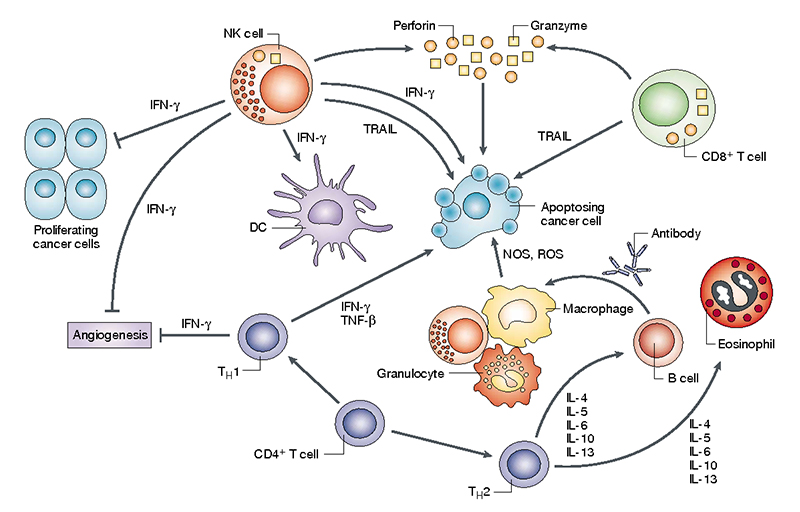
Cytokines are secreted or membrane-bound proteins that regulate the growth, differentiation, and activation of immune cells. They are important molecules involved in the immune response generated against any infection. They are the main regulators of both branches of the immune response, i.e., innate and adaptive, that help in the communication of the cells of the immune system in both an autocrine and paracrine manner. There has been a great deal of interest in cytokine research due to their ability to recognise and kill cancer cells.
Cytokines are released in the body as a response to several stress factors, like infection, carcinogen-induced injury, and inflammation. They seem to play a critical role in the field of cancer. They can also function to inhibit cancer progression and development. Alternatively, cancer cells also respond to cytokines derived from the host, which promote their growth, attenuate apoptosis, and facilitate invasion and metastasis. Inflammatory conditions in some tissues increase the risk of cancer. Cytokines and chemokines are components of an intensive dialogue promoting angiogenesis, metastasis, subversion of adaptive immunity, and changing responses to hormones and chemotherapeutic agents.
Cancer is a hyperproliferative disorder characterised by cellular transformation, apoptosis dysregulation, uncontrolled cellular proliferation, invasion, angiogenesis, and metastasis. Clinical and epidemiologic research has found a strong cross-link between chronic infection, inflammation, and cancer. For example, there is a strong link between alcohol abuse and hepatitis, pancreatitis, and cancers of these organs. Cigarette smoking, asbestos, and silica exposure are associated with lung inflammation and lung cancer; inflammatory bowel disease is associated with colon cancer; and chronic viral hepatitis is further associated with liver cancer. These findings suggest the role of chronic inflammation in tumour initiation, proliferation, and progression. Cells such as macrophages, T cells, and natural killer cells are involved in the inflammatory process. Tumor-associated macrophages (TAMs) and T cells are frequently the prominent leukocytes found in a tumor. Many studies show that acute inflammation caused by tumor-infiltrating host leukocytes does not activate normal immunoprotective mechanisms that lead to cancer eradication. Instead, proinflammatory mediators that are excessively and chronically produced are thought to contribute to tumour promotion and progression.
There is a balance between antitumor immunity and tumor-originated proinflammatory activity, which weakens antitumor immunity. Tumor cells undergo immune escape and grow rapidly when antitumor activity is weaker than tumor-mediated immunosuppressive activity. On the contrary, when antitumor immunity is stronger than tumor-mediated immunosuppressive activity, tumor cells are eliminated. Chronic inflammation results in increased tumour growth, accelerated tumour progression, invasion of surrounding tissues, angiogenesis, and metastasis. The inhibition of the NF-kB kinase/NF-kB signalling pathway, which is activated by many secreted proinflammatory cytokines, provides a critical molecular link between inflammation and tumor growth and progression. NF-kB is a transcription factor that regulates the expression of many genes, the products of which can suppress tumor cell death, stimulate tumour cell cycle progression, improve epithelial-to-mesenchymal transition, which plays an important role in tumor invasiveness, and provide newly emerging tumors with an inflammatory microenvironment that promotes tumour progression and invasion of surrounding tissues.
Cancer-related cytokines are IL-1, IL-6, IL-10, IL-12, IL-23, TNF-α, TGF-β, VEGF, and others. All of them have critical functions in cancer development. IL-1 is important for the tumor invasion and angiogenesis, IL-6 for chemically induced lymphomas and also has a role in pathogenesis of malignancy, IL-10 aids in the inhibition of cancer antigen presentation, IL-12 inhibits chemical carcinogenesis, TNF-α helps in the induction of tumor-cell apoptosis; activates endothelium and granulocytes, TGF-β inhibits T-cell effector function. Along with this, it has been shown that the VEGF family plays an important role in tumor angiogenesis, is overexpressed in a large percentage of solid tumors, and is closely associated with a poor prognosis. VEGF is not only critical for tumor angiogenesis but also an important factor produced by solid tumors to inhibit the recognition and destruction of tumor cells by the immune system. There are a variety of chemokines like CCL2, CXCL12, CXCL8, CXCL1, CXCL13, CCL5, CCL17, and CCL22 that have been observed in neoplastic tissues as a product of either tumor cells or stromal elements. CXCL1 and related molecules [CXCL2, CXCL3, CXCL8, or interleukin-8 (IL-8)] have a key role in the progression of melanoma by stimulating neoplastic growth, inducing angiogenesis, and promoting inflammation.
Role of TNF-α
TNF-α has a well-established role in chronic inflammatory diseases, and its tumor-promoting effects have already been demonstrated. TNF-α, produced by tumor cells or inflammatory cells in the tumor promoting microenvironment, promotes tumor cell survival by inducing genes that encode NF-kB-dependent antiapoptotic molecules. Macrophages phagocytoze asbestos and then release TNF-α in asbestos-induced malignant mesothelioma. This TNF-α promotes cell survival, reducing asbestos-induced cytotoxicity and increasing the pool of asbestos-damaged mesothelial cells susceptible to malignant transformation. TNF-α has also been proposed to contribute to tumour initiation by stimulating the production of genotoxic molecules such as nitric oxide and reactive oxygen species, which can cause DNA damage and mutations. TNF-producing genetic polymorphisms are linked to an increased risk of bladder cancer, hepatocellular carcinoma, gastric cancer, and breast cancer, as well as a poor prognosis in a several haematological malignancies. TNF-α also promotes angiogenesis and metastasis, as well as impairing the immune system by suppressing T cell responses and the cytotoxic activity of activated macrophages. TNF-αhas also been found to play a tumor-promoting role in cholestatic liver cancer, which develops as a result of chronic liver inflammation in mice lacking the drug and phospholipid transporters. During the tumor proliferation stage, treatment with a TNF- α specific neutralising antibody resulted in apoptosis of transformed hepatocytes and failure to progress to hepatocellular carcinoma.
Interleukin 6
IL-6 is a potent inflammatory cytokine that is a key growth-promoting and antiapoptotic factor. Activation of the IL-6 receptor causes phosphorylation of the STAT proteins STAT1 and STAT3. STAT3 promotes malignant cell proliferation, whereas STAT1 inhibits tumour cell growth. The majority of IL-6 target genes are involved in cell cycle progression and apoptosis suppression, both of which contribute to tumorigenesis. It has been proposed that IL-6 plays a critical role in the pathogenesis of Kaposi sarcoma. Other studies have found a link between circulating IL-6 and an increased risk of developing Hodgkin lymphoma.
Interleukin 17
Th17 cells are distinguished by the production of IL-17, which is involved in inflammatory responses. The production of IL-17 is dependent on STAT3 activation, which is triggered by IL-23. IL-17 causes immune cells to migrate to peripheral tissues, a response that requires NF-kB activation following IL-17 receptor engagement. Many proinflammatory factors, including TNF-α and IL-6, are also activated by IL-17, implying that interleukin plays an important role in localising and amplifying inflammation. Furthermore, TNF-α and IL-6, both produced by Th17 cells, work in tandem with IL-17 to increase the production of proinflammatory mediators. In immunocompetent mice, there is evidence that IL-17 is involved in tumour surveillance.
Interleukin 12 & 23
Phagocytes are the primary producers of IL-12 and IL-23. Both receptors are primarily expressed on T cells, NK cells, monocytes, macrophages, and DCs. STAT protein is activated by both cytokines. IL-12 has been shown to inhibit tumorigenesis and induce regression of established tumours in mouse models of cancer by activating Th1 adaptive immunity and increasing interferon production, which has a direct toxic effect on cancer cells and antiangiogenic activity. IL-23 can boost memory T-cell proliferation as well as activated T-cell production of IFN and IL-12. It can, however, drive Th17-mediated responses, induce IL-17 and TNF- α production by macrophages, and promote end-stage inflammation, which can lead to cancer development. The growth of transplanted tumors was found to be restricted in hosts lacking IL-23.
Interleukin 10
IL-10 is immunosuppressive and anti-inflammatory; its effects are diametrically opposed to those of IL-6. IL-10 inhibits NF-kB activation and, as a result, the production of proinflammatory cytokines such as TNF-α, IL-6, and IL-12. IL-10 has also been shown to suppress angiogenesis and modulate apoptosis during tumour regression
There are several cytokines that play a critical role in the pathogenesis and management of cancer in different ways. The drugs acting as agonists or antagonists to some of these cytokines may represent a new hope for cancer therapy.
Bioelsa offer highly stable and finest quality of ELISA kits for the detection of proinflammatory cytokines and there by support cancer research world-wide.